Organic Molecules:
The molecular absorptions within the UV-VIS region depend on the electronic structure of the molecule. A wavelength of the radiation absorbed through an organic molecule is determined via the difference in energy among the ground state and the several excited electronic states of the molecule. You would recall from your previous knowledge of bonding within organic molecules in which the constituent atoms are bonded by σ and π bonds. Further, these have nonbonding electrons on the atoms such as, N, O, S and halogens etc. There are a number of transitions possible including the bonding and the nonbonding electrons. The generalized molecular orbital energy level diagram and probable transitions for organic compounds is given in Figure.
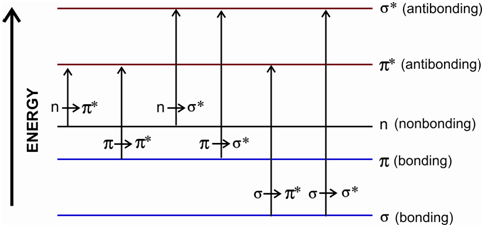
Figure: Generalised molecular orbital energy level diagram and possible transitions for organic compounds
As a rule, the transitions occur from the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO) in a molecule. Within most of the organic elements, a bonding and sometimes the nonbonding orbitals also are filled and the antibonding orbitals are null. Of the six probable transitions implies within the figure, just the two of the lowest energy ones (n → π ∗ and π → π ∗) could be achieved through the energies available in the 200 to 800 nm region. Let us learn about these two in the following paragraph.