Inorganic Species:
A huge number of inorganic salts holding atoms along with electrons in d-orbitals give weak absorption bands in the visible range. The ions and the complexes of the components of the first two transition series belong to this group and are colored. The color of these species is due to transitions amongst d-orbitals. The complex creation of these ions along with solvent molecules or with other ligands lifts the degeneracy of the five d-orbitals. As a result, these split within groups having different energies. The electronic transitions from the lower energy d-orbitals to higher energy d-orbitals are responsible for the observed color. These transitions are known as d-d transitions. A blue color of the aqueous solutions of copper sulphate and the violet color of potassium permanganate are a few of the examples.
The ions of the lanthanides and actinides are also colored; therefore, these include f-f transitions. The nature of spectrum within case of these ions is different since the f-electrons are associatively less affected through external influences because of shielding through the occupied orbitals of higher principal quantum number. These ions absorb the radiation of ultraviolet and visible region in narrow bands. The representative visible spectra of the ions of transition and inner transition elements are provided in Figure.
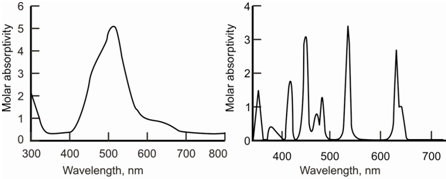
Figure: Representative absorption spectra for the a) transition and b) inner transition element ions