Charge Transfer Complexes:
Several a times a given compound that is transparent in the UV region begins absorbing after interacting along with another species. This will happens if one of the species has an electron donor group and the interacting species has an electron acceptor group. While the two species bind to another, the resulting species is intensely coloured. This is because of the formation of a complex among the two species. Such a complex is known as charge transfer complex. For instance, the blood red color of the complex ion, thiocyanatoiron (III) ion, Fe (SCN) 2+ is because of the formation of a charge transfer complex.
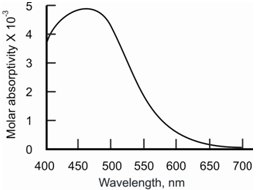
Figure: The charge transfer spectrum of the complex ion, Fe (SCN)2+
The radiation absorbed through this product or complex causes the transfer of an electron from an orbital on the donor to an orbital of the acceptor. This has been schematically described in Figure. The transitions in the donor and the acceptor species individually are of high energy and absorb outside the UV-VIS region. Thus, in the complex, the energies of the orbitals are such that the HOMO to LUMO transition is of much lower energy and falls within the visible region.