Structure and bonding
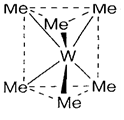
Alkyl ligands create metal-carbon σ bonds. Frequently they take place in conjunction with another organic ligands or CO, but can be notice on their own, like in tungsten hexamethyl (1), and in [Ti(CH2SiMe3)4] in which the bulky groups are useful in stabilizing the compound. Compounds along with H attached to β carbons (the nomenclature being M-Cα-Cβ-Cγ) aimed to be unstable to β-hydride removal of an alkene fragment, that is discussed below. The surprising structure of (1), trigonal prismatic (D3h) than octahedral as observe in WCl6, has been recognized to the orientation of d orbitals presented for σ bonding. Only two d orbitals (the eg set) can be involved in an octahedral complex, but four in the trigonal prismatic structure. (Not like the WMe6, WCl6 also have some degree of W-Cl π bonding, that can involve the other d orbitals (t2g) in octahedral geometry)
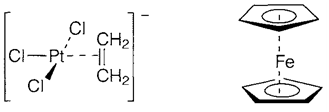
Alkylidene and alkylidyne ligands need metal-carbon π bonding besides σ. This is dissimilar, though, from π complexes in which bonding include only the π orbitals of alkene or aromatic ligands. Instances are the ethene complex [(η2-C2H4)PtCl3]- (2) found within Zeise's salt, and the 'sandwich compound' ferrocene [Fe(η5-C5H5)2] (3). The Dewar-Chatt-Duncanson model of bonding in ethene complexes is displayed in diagram 1 and is similar to the σ-donor-π-acceptor explanation of the bonding in carbonyl complexes. In the current case the 'σ-donor' character comes by the occupied bonding π MO of ethene (diagram 1a), back donation (diagram. 1b. involving the empty π* antibonding MO. The comparative degrees of donor or acceptor behavior rely on the compound.
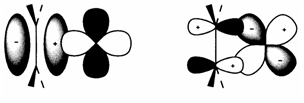
Fig. 1. Dewar-Chatt-Duncanson model for bonding in π complexes of C2H4.
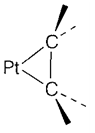
With strongly electron-withdrawing alkenes like C2F4 or C2(CN)4 there is a large amount of back donation, that weakens the C-C bond thus that its length is identical to that of a single bond. The geometry of the ligand then too changes by the planar configuration related with sp2 hybridization towards a nonplanar form more characteristic of singlebonded sp3. The product (4) can be viewed like a metallocyclic compound along with two M-C σ bonds.
Bonding in sandwich compounds like ferrocene arises via interaction of the delocalized π MOs of the ring with orbitals of the metal, and not treated in a localized way. Like in alkenes, both donor and acceptor interactions are included. Other ligands like CO can be exist, like in the 'piano-stool' structure 5 or the metalmetal bonded dimer 6.
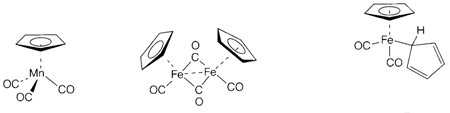
The 18-electron rule can be a helpful guide to stable organometallic compounds, particularly when π-acceptor ligands are exists, even though it has the limitations considered to in Topic H9. Compounds 3, 5 and 6 follow this rule, but 1 with no π bonding ligands has an electron count of just 12. Metallocenes [M(η5-C5H5)2] are recognized for the 3d series elements V-Ni, with 15-20 valence electrons, correspondingly. Ferrocene (M=Fe with 18 electrons) is certainly the most stable of these, cobaltocene (M=Co with 19 electrons) being a extremely strong reducing agent which easily creates the 18-electron ion [Co(η5-C5H5)2]+. Compounds along with more than 18 valence electrons are rare, and so one canunderstand the unusual structure of [Fe(η5-C5H5)(η1-C5H5)(CO)2] (7), as two pentahapto ligands would provide an electron count of 22. Reactions of organometallic compounds frequently include 16-electron intermediates created through the loss of one ligand (example CO) from an 18-electron parent compound.