Ligand classification
Organometallic compounds along with metal-carbon bonds are created through almost all metallic elements, but those of transition metals depict diversity with no parallel in main groups. Cyanide and Carbonyl ligands are not referred organic, even though they may also be exists in organometallic compounds with other π-acceptor ligands like phosphines.
Table 1 depicts a selection of the ligands that are found in organometallic compounds of transition metals, classified as per the two properties.
- The hapticity is the number of carbon atoms directly bonded to the metal. With some ligands this can differ; for instance, cyclopentadienyl could be η1-C5H5, η3-C5H5 or (most frequently) η5-C5H5 (pronounced'trihapto', 'monohapto', etc.).
- The number of electrons the ligand that are contributes to the metal-carbon bonding is known as the electron number. This is helpful for applying the 18-electron (EAN) rule. Ligands are occupied to be neutral species even if they
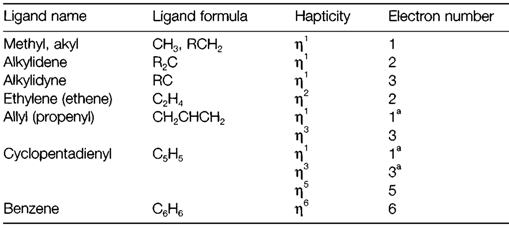
Not common bonding arrangements are termed as stable anions (example C5H5, not C5H5-). For ligands of variable hapticity the electron number frequently changes consequently, but electron number is not all the time equivalent to the hapticity, as can be seen with η1 ligands, in which the electron number can change from one to three.