Insertion and elimination
Among the several reactions of organometallic compounds, ones including insertion and elimination of ligands are significant in applications to synthesis and catalysis. An instance of a carbonyl insertion is:
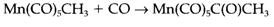
Where a Mn-CH3 bond is replaced through Mn-C(O)-CH3. The term is misleading because it is established through isotopic labelling that the incoming CO is not the one inserted. The 1st step is a reversible alkyl migration that is leading to a 16- electron intermediate that then picks up other CO molecule as displayed in 8.
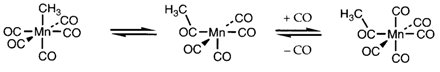
Fig. 2. Reaction steps involved in the catalytic Monsanto acetic acid process.
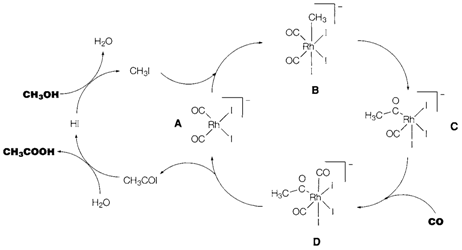
Several other unsaturated ligands can 'insert' into M-C or M-H bonds; for instance, alkanes like in:
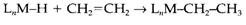
Such types of reactions are frequently reversible, the backwards procedure leading to elimination of a ligand. The opposite of alkene insertion is the β-hydride elimination reaction considered to above.
Organometallic compounds are used extensively like homogeneous catalysts in the chemical industry. For instance, if the alkene insertion reaction carries on with further alkene inserting into the M-C bond, it can create the basis for catalytic alkene polymerization. Another catalytic cycle might involve oxidative addition and reductive elimination steps like explained in Topic H9. Figure 2 depicts the steps concerned in the Monsanto acetic acid process, that performs the conversion
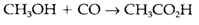
In the catalytic cycle on the right side, the 16-electron species A goes through oxidative addition of CH3I to form B.
Carbonyl insertion then continues through C to give D, that regenerates A through reductive elimination of CH3COI. The organic steps on the left side of diagram 2 can be varied to provide dissimilar overall reactions, for instance, converting CH3CO2CH3 into (CH3CO)2O.