Nucleophilic centers:
By using the above guidelines, the nucleophilic and electrophilic centers of the common functional groups can be recognized in which atoms comprising a slightly negative charge are nucleophilic centers and atoms comprising a slightly positive charge are electrophilic centers.
Not each nucleophilic and electrophilic center is of equal significance. For instance, a nitrogen atom is much more nucleophilic as compared to an oxygen atom. As well halogen atoms are extremely weak nucleophilic and will not generally react with electrophiles if there is a stronger nucleophilic center present. Hydrogen atoms connected to halogens are more electrophilic as compared to the hydrogen atoms attached to oxygen. Hydrogen atoms attached to nitrogen are extremely weak electrophilic.
Taking this into consideration, a number of functional groups are more similarly to react like nucleophiles whereas some functional groups are more likely to react like an electrophiles. For instance, amines, alcohols and ethers are more likely to react like nucleophiles, because they comprise strong nucleophilic centers and weak electrophilic centers. Alkyl halides are more probable to react like electrophiles because they encompass strong electrophilic centers and weak nucleophilic centers. Aldehydes and ketones can react like nucleophiles or electrophiles because both of the electrophilic and nucleophilic centers are strong.
A number of functional groups contain various nucleophilic and electrophilic centers. For instance, carboxylic acids and their derivatives fall into this class and thus there are various possible centers in which a nucleophile or an electrophile could react.
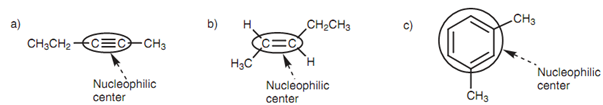
Figure: Nucleophilic centers in (a) an alkyne; (b) an alkene; (c) an aromatic compound.