Proton Nuclear Magnetic Resonance Spectroscopy
Of all the spectroscopic methods, nuclear magnetic resonance (nmr) spectroscopy is the very much helpful in ascertaining the structure and stereochemistry of organic compounds. Nmr spectroscopy observes the nuclei of atoms in a molecule, and one of the most helpful makes of nmr is the detection of hydrogen atoms. The nucleus of a hydrogen atom is a single proton and thus the method is also known as proton nmr. A proton spins around its axis, and when a charged body spins, a magnetic field is set up that can be presented through a magnetic dipole moment. Within normal conditions, the protons and their magnetic moments are randomly orientated and thus there is no overall magnetic field.
Though, the condition changes if an external magnetic field is applied to the sample. In diagram 1b and c an external magnetic field has been applied in the direction of the z-axis. This field interacts along with the magnetic moment of the nucleus, that forcing the nucleus to spin in only two possible orientations. In the figure the nucleus is spinning such that the magnetic moment is pointing in roughly similar direction like the field.
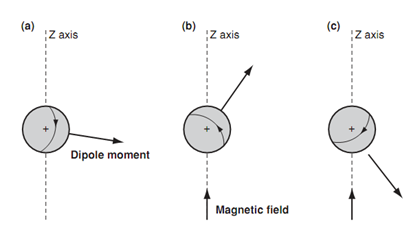
Figure: Orientation of a proton's magnetic dipole moment; A) No magnetic field B) Alignment with magnetic field C) Alignment against magnetic field.