Spectrum of acetone:
n → π ∗ transitions: These transitions are observed in molecules holding lone pairs or nonbonding electrons. Within such transitions one of the nonbonding electrons might be excited within an empty π orbital. The energies needed for these transitions are lower than that for π → π ∗ transitions and result in the absorption in the ultraviolet and visible region. The presence of atoms or groups holding n = electrons, could cause remarkable changes in the spectrum. Therefore, nitrogen, sulphur and halogens tend to move absorption to higher wavelengths.
The π → π∗ transitions are commonly intense although the n → π ∗ transitions are weak. For instance, acetone, exhibits a high intensity π → π ∗ transition at 195 nm and a low intensity n → π ∗ transition at 274 nm within its absorption spectrum.
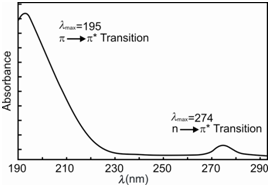
Figure: UV spectrum of acetone showing the π → π∗ and n → π ∗ transitions
Thus, only those molecules are likely to absorb light in the 200 to 800 nm region that contain π-electrons and might also have atoms along with non-bonding electrons. Such groups that absorb light in the UV-VIS region are referred to as chromophores.