Ethers, epoxides, and thioethers:
Ethers
Ethers are a division of organic compounds which consist of an ether group - an oxygen atom linked to two alkyl or aryl groups - of common formula R-O-R'. A typical instance is the solvent and anesthetic diethyl ether, generally referred to simply as "ether" (CH3-CH2-O-CH2-CH3). Ethers are general in organic chemistry and pervasive in biochemistry, since they are common linkages in carbohydrates and lignin.
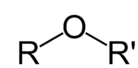
Figure: General structure of Ethers
Epoxide
An epoxide is cyclic ether along with three ring atoms. This ring almost defines an equilateral triangle that makes it highly strained. The strained ring forms epoxides more reactive as compared to other ethers. Simple epoxides are entitled from the parent compound ethylene oxide or oxirane, like in chloromethyloxirane. Since a functional group, epoxides feature the epoxy prefix, like in the compound 1,2-epoxycycloheptane that can also be termed as cycloheptene epoxide or simply cycloheptene oxide.
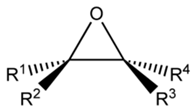
Figure: Generic Epoxide
Thioethers
A functional group in organosulfur chemistry with the connectivity C-S-C is known as a thioether. Like several other sulfur-containing compounds, volatile thioethers have foul odors.
A thioether is identical to ether except that it consists of a sulfur atom in place of the oxygen. The oxygen's and sulfur's grouping in the periodic table suggests that the chemical properties of ethers and thioethers are somewhat similar.
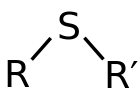
Figure: General structure of Thioethers