Carboxylic acids and carboxylic acid derivatives
An organic acid characterized by the existence of at least one carboxyl group is known as Carboxylic acids. The common formula of a carboxylic acid is R-COOH, in which R is some monovalent functional group. A carboxyl group / carboxy is a functional group that contains a carbonyl (RR'C=O) and a hydroxyl (R-O-H), that has the formula -C (=O) OH, generally written like -COOH or -CO2H.[2]
Carboxylic acids are Brønsted-Lowry acids since they are proton (H+) donors. They are the most general type of organic acid. Between the simplest instances are formic acid H-COOH that occurs in ants, and acetic acid CH3-COOH, which provides vinegar its sour taste. Acids with two or much more carboxyl groups are known as dicarboxylic, tricarboxylic, etc. The very simple dicarboxylic instance is oxalic acid (COOH)2 that is just two connected carboxyls. Mellitic acid is an instance of a hexacarboxylic acid. Other significant natural instances are citric acid (in lemons) and tartaric acid (in tamarinds).
Salts and esters of carboxylic acids are known as carboxylates. While a carboxyl group is deprotonated, the conjugate base of it, a carboxylate anion is made. Carboxylate ions are resonance stabilized and this improved stability makes carboxylic acids much more acidic as compared to alcohols. Carboxylic acids can be seen like reduced or alkylated forms of the Lewis acid carbon dioxide; within some conditions they can be decarboxylated to yield carbon dioxide.
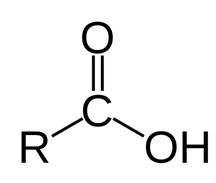
Figure: Structure of Carboxylic acids