Aromatics:
The other wide class of hydrocarbons is the aromatic hydrocarbon. Rather than being arranged within straight chains, since the aliphatics are, those are cyclic formations like as in benzene. A derivative of cyclic hydrocarbons has pleasant (sometimes toxic) odors. Benzene in rubber cement is a familiar odor. A cyclic compound has alternating single - double bonds as described in Figure.
Aromatic hydrocarbons are incredibly stable chemically, and act extremely much such as alkanes. That will undergo substitution reactions rather than further.
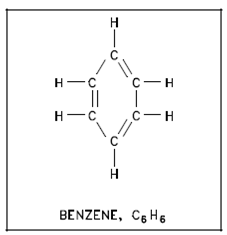
Figure: Aromatic