Optogenetic protocols
An extremely promising optogenetic device is channelrhodopsin-2 (ChR2), an algal protein. It responds to blue light by opening a nonspecific cation conductance. The resulting inward Na+ current excites any neuron that has incorporated ChR2 into its membrane. In addition to its use as an actuator fluorescently labeled ChR2 reveals light-stimulated axons and synapses in intact brain tissue. This has been used to study the molecular events in spike timing-dependent plasticity. In addition ChR2 has been used to map long-range cortico-cortical connections in the brain, and to map the spatial location of specific inputs on the dendritic tree of individual cortical pyramidal neurons.
The gene archaerhodopsin-3 (Arch) codes for an outward proton pump. When virally expressed in mouse cortex and illuminated with yellow light it almost completely silences those neurons that express the protein. Arch spontaneously recovers from light-dependent inactivation unlike light-driven chloride pumps which enter long-lasting inactive states in response to light. This means that Arch could mediate several cycles of optical silencing during an experiment. Expressed in specific cells this could be used to investigate their role in any particular neuron function by temporarily inhibiting the cells. Unlike gene knockout experiments, optogenetic silencing can be transient and can be repeated any number of times, allowing animals to be used as their own controls. Another actuator protein, Mac, a light-driven proton pump derived from a fungus, silences neurons when illuminated by blue light. Using Arch and Mac together allows silencing of two neural populations independently with two different wavelengths of light. A typical optogenetic protocol is shown in below Figure.
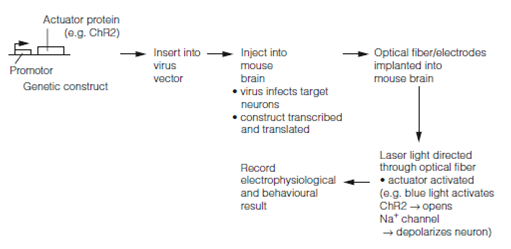
Figure: A typical optogenetic protocol.