Separation of six steroids:
In many cases when the bands overlap, the selectivity factor is made larger by adjusting to a suitable level. Such a change is conveniently made by changing the chemical nature of the mobile phase as typically shown for the separation of six steroids in Figure by reverse-phase chromatography following a four solvent optimization procedure consisting of methanol, acetonitrile, tetrahydrofuran and water. It was developed for finding a suitable solvent system to resolve a given mixture in a minimum possible time. Three compatible solvents were used to adjust the strength of the mixture to yield a suitable value of ' . The first two chromatograms in (a) and (b)
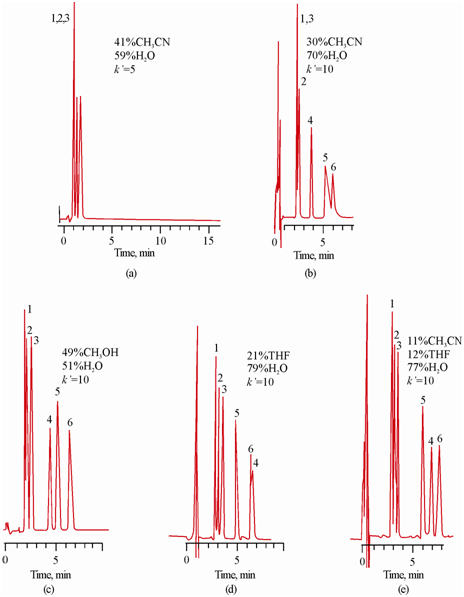
Figure: Choice of mobile phase on the selective separation of six steroids using 5 µm C8 bonded reversed phase particles. Peak ; 1. Prednisone, 2. Cortisone, 3. Hydrocortisone, 4. Dexamethasone, 5. Corticosterone, 6. Cortoexolone. Effect of % water to adjust ′ in (a) and (b). Further separation factor α is varied at constant ′ in (b), (c), (d) and (e)
show the results from initial experiments to determine minimum value of ′ which is estimated to be 10. However, it is observed that α values for components 1 & 3 and that of 5 & 6 do not yield satisfactory resolution. In further experimentation for finding better α values, water was added to get ′ = 10. It is observed that results of methanol/water and tetrahydrofuran/water in (c) and (d) show better resolution. A mixture of acetonitrile, THF and water was also attempted as shown in (e) where it is found to be the best mobile phase for the separation of six steroids in a mixture.