Atom De Broglie Model:
The dual behaviour of photons inspired De Broglie to suggest a model of the atom. The experiments on interference, diffraction and polarization established the wave nature of light. The experiments on photo-electric effect established the corpuscular nature of light. Einstein's equation E = mc2, where E is the energy, m is the mass and c is the velocity of light, gives the relation of conversion of mass into energy. De Broglie noted that the corpuscular properties were more obvious for very energetic light i.e. light of shorter wavelength. Thus, the light particles known as a photon has corpuscular as well as wave nature.
Thinking on the same lines, De Broglie suggested that particles like electrons have also a wave nature.
For a particle, Einstein's equation is
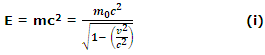
Here m0 is the rest mass.
The energy of a wave of frequency v according to Planck is
E = hv (ii)
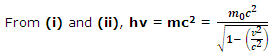
But, vλ = c

for an electron of velocity v
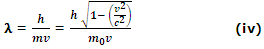
For electrons, the wavelength is longest, while for protons it is smaller than the electrons. If the value of h, m and v are substituted in equation (iv), λ is found to be very small.
Thus, the De Broglie model of an atom has electrons in various orbits and the electrons behave as matter waves of wavelength λ = h/mv. The electron exists as a standing wave in each orbit. The energy levels and 'orbits' of the Bohr's postulates. The electrons can be only in those orbits whose circumference can contain the complete wave of the electron or the length of the orbit is a whole number multiple of the wave length.
The electron cannot be in an orbit whose length is not a whole number multiple of the wavelength. The necessary condition for the electron in a particular orbit can be calculated mathematically. The length of the orbit of radius r = 2πr and 2πr = nλ, where n is a whole number,
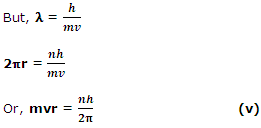
Equation, (v) is in agreement with the Bohr's quantization hypothesis. Thus, the De Broglie model seems to be more exact and the electrons are matter waves in various orbits round the nucleus. Davisson and Germer experiment on the diffraction of electrons demonstrated the fact that the material particles like electrons exhibit a wavelength given by the De Broglie equation, λ = h/mv .