R and S nomenclature:
The structure of an enantiomer can be fixed through the R and S nomenclature, ascertained by the Cahn-Ingold-Prelog rules. The instance shows how the nomenclature is worked out. Very first, the atoms directly related to the asymmetric center and their atomic numbers are identi?ed. After day, you provide the attached atoms a priority based on their atomic numbers. In this instance, there are two carbon atoms with similar atomic numbers and thus they cannot be given a priority.
While this occurs, the next stage is to move to the next atom that has the highest atomic number. The meaning of this is moving to oxygen for one of the carbons and to hydrogen for another. The oxygen has the higher priority and thus this substituent takes priority over another. One time the priorities have been settled, the structure is redrawn like that the group of lowest priority is positioned 'behind the page'. In this instance (Fig.),
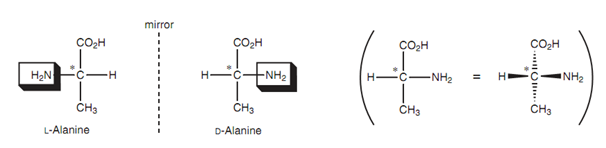
Figure: Fischer diagrams of L-alanine and D-alanine.
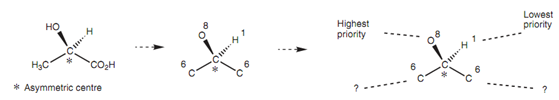
Figure: Assigning priorities to substituents of an asymmetric center.