Asymmetric molecules:
A molecule like chloroform (CHCl3) is tetrahedral and there is just only one way of fitting the atoms together. This is not the matter for a molecule like lactic acid.
There are two methods of constructing a model of lactic acid, like that the two structures acquired are non super-imposable and cannot be inter converted with no breaking covalent bonds. Because such, they present two different molecules that are configurational isomers. The variation among the two possible molecules lies in the way the substituents are connected to the central carbon. This can be presented by the below figures in which the bond to the hydroxyl group emerges of the page in one isomer but goes into the page in another isomer. The lactic acid's 2 isomers are mirror images. A molecule that exists as two non super-imposable mirror images has optical activity if just only one of the mirror images is exist.
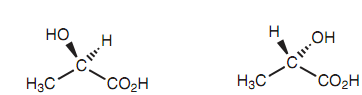
Figure: Lactic acid.
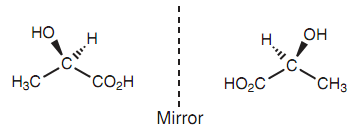
Figure: Non super-imposable mirror images of lactic acid.
Lactic acid presents as two non super-imposable mirror images since it is asymmetric - in other words it lacks symmetry. Asymmetric molecules can as well be known as chiral, and the capability of molecules to exist because two optical isomers are termed as chirality. Actually, a molecule does not have to be completely asymmetric to be chiral. Molecules consisting of a single axis of symmetry can also be chiral.