Asymmetric carbon centers:
A simple way of recognizing most chiral molecules includes identifying what are termed as asymmetric carbon centers. This works for several chiral molecules, but it is significant to realize that it is not foolproof and that there are various cases in which it will not work. For instance, some chiral molecules have no asymmetric carbon centers, and a number of molecules comprising more than one asymmetric carbon center are not chiral.
Generally, a compound will have optical isomers if there are four dissimilar substituents connected to a central carbon. In such types of cases, the mirror images are non super-imposable and the structure will present because two con?gurational isomers termed as enantiomers. The carbon center that consists of these four different substituents is termed as a stereogenic or asymmetric center. A solution of every enantiomer or optical isomer is able of rotating plane-polarized light. One enantiomer will rotate plane-polarized light clockwise where as the other (the mirror image) will rotate it counterclockwise by similar amount. A mixture of the two isomers (a racemate) will not rotate plane-polarized light at all. In all other ways, the two isomers are similar in physical and chemical properties and are hence indistinguishable. The asymmetric centers in the molecules displayed in the below figure have been make out with an asterisk. The structure that is lacking the asymmetric center is symmetric or achiral and does not comprise optical isomers. A structure can as well have more than one asymmetric center.
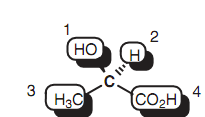
Figure: Four different substituents of lactic acid.
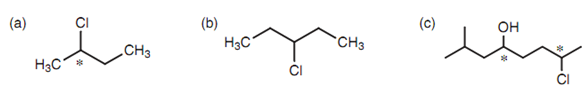
Figure: Chiral and achiral structures: (a) chiral; (b) achiral; (c) chiral.
Asymmetric centers are just only possible on sp3 carbons. A sp2 center is planar and cannot be asymmetric. Likewise, a sp center cannot be asymmetric.