Allenes and Spiro compounds:
Not each chiral molecule has asymmetric centers. For instance, a number of substituted allenes and Spiro structures comprise no asymmetric center but are still chiral. The substituents at either end of the allenes are in dissimilar planes, and the rings in the Spiro structure are at right angles to each other. The allenes and the Spiro structures' mirror images are non super-imposable and are enantiomers.
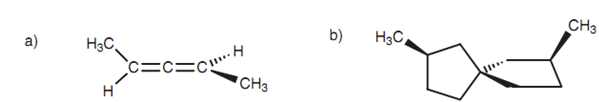
Figure: (a) Allene; (b) Spiro structure.
A better rule for ascertain whether a molecule is chiral or not is to study the symmetry of the molecule. A molecule will be chiral if it is asymmetric (that is has no elements of symmetry) or if it has no more than one axis of symmetry.