Current - voltage curves:
Cells which exhibit non-linear relationship are said to be polarised and the degree of polarisation is given through overvoltage or overpotential. Polarisation needs the application of a potential greater than the theoretical value to provide a current of the expected magnitude. Therefore an overpotential of - 0.04 V is needed to acquire a current of 0.06 A in the electrolytic cell as describes in Figure. In a galvanic cell, the output cell potential decreases through about 0.03 V i.e. the overvoltage is 0.03 V as described in Figure. Remember which in each case the overvoltage is negative.
Therefore, for an electrolytic cell affected through overvoltage, Eq. then becomes,
Eapplied = Ecell - IR - overvoltage
In easy terms, the overvoltage is the potential difference among the theoretical cell potential determined through Eq. and the actual cell potential at a provided level of current. It could be calculated through Eq. Instead polarization is the departure of the electrode potential from its theoretical value on passage of current.
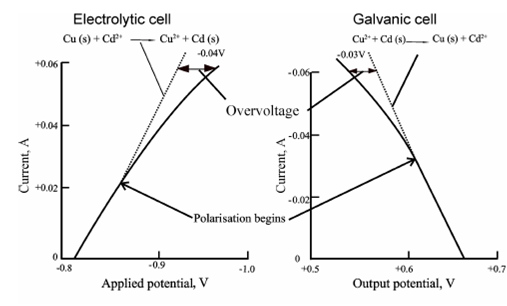
Figure: Current/voltage curves for (a) an electrolytic and (b) a galvanic cell