Walden inversion of an asymmetric center:
Though, the 'umbrella' is pointing in a dissimilar direction in the alcohol product as compared to the alkyl halide. The meaning of this is that the 'umbrella' has been turned inside out during the mechanism. Other words, the carbon center have been 'inverted'. Transition state is the halfway house in this inversion.
There is not any way of telling whether inversion has occurred in a molecule like iodomethane, but proof of this inversion can be acquired via looking at the nucleophilic substitution of asymmetric alkyl halides along with the hydroxide ion.
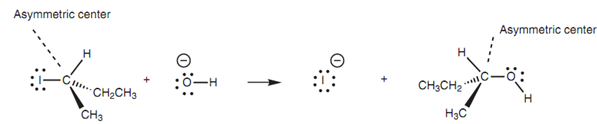
Figure: Walden inversion of an asymmetric center.
Calculating the optical activity of both the alkyl halide and the alcohol permits the configuration of each enantiomer to be recognized. This in turn illustrates that inversion of the asymmetric center occurs. This inversion is termed as the 'Walden Inversion' and the mechanism is termed as the SN2 mechanism. Here the SN stands for 'substitution nucleophilic'. The 2 signifies the rate of reaction is second order or bimolecular and depends upon both the concentration of the nucleophile and the concentration of the alkyl halide. The SN2 mechanism is probable for the nucleophilic substitutions of primary and secondary alkyl halides, but is hard for tertiary alkyl halides. We can draw a common mechanism to account for a range of alkyl halides and charged nucleophiles.