Walden inversion:
Additionally, the hydroxide ion has to acquire access to the reaction center - the electrophilic carbon. There is much more room to attack from the 'rear' because the large iodine atom blocks approach from another side. Finally from an orbital point of view, it is proposed that the orbital from the incoming nucleophile begins to overlap with the empty antibonding orbital of the C-X bond.
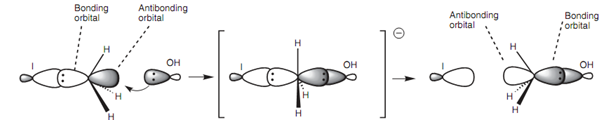
Figure: Orbital interactions in the SN2 mechanism.
Since this interaction increases, the bonding interaction among the carbon and the halogen decreases until a transition state is arrives at where the incoming and outgoing nucleophiles are both partially bonded. The orbital geometry needs the nucleophiles to be on opposite sides of the molecule.
A 3rd interesting feature about this mechanism regards the three substituents on the carbon. Both of the iodide and the alcohol product are tetrahedral compounds with the three hydrogens creating an 'umbrella' shape with the carbon.
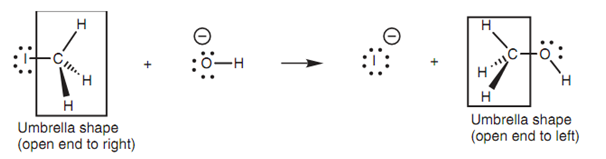
Figure: Walden inversion.