SN1 Mechanism:
While an alkyl halide is dissolved in a protic solvent like ethanol or water, it is exposed to a non basic nucleophile (that is the solvent molecule). Within these conditions, the order of reactivity to nucleophilic substitution changes radically from that observed in the SN2 reaction, like that tertiary alkyl halides are much more reactive then secondary alkyl halides, along with primary alkyl halides not reacting at all. Clearly other mechanism must be involved. As an instance, we shall think the reaction of 2-iodo-2-methylpropane with water. Here, the rate of reaction depends upon the concentration of the alkyl halide alone and the concentration of the attacking nucleophile comprises no effect. Visibly, the nucleophile has to be present if the reaction is to happen, but it does not matter either there is one equivalent of the nucleophile or an excess. Because the reaction rate only depends upon the alkyl halide, the mechanism is termed as the SNl reaction, in which SN stands for substitution nucleophilic and the 1 depict that the reaction is first order or unimolecular, i.e., only one of the reactants affects the reaction rate.
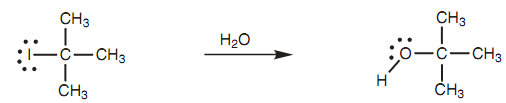
Figure: Reaction of 2-Iodo-2-methylpropane with water.