Mechanism - neutral nucleophiles:
Acid chlorides are adequately reactive to react with not charged nucleophiles. For instance, ethanoyl chloride will react with methanol to provide an ester shown in figure.
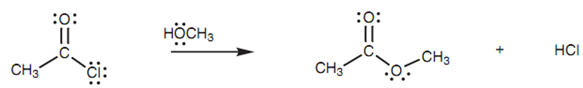
Figure: Ethanoyl chloride reacting with methanol to form methyl ethanoate.
Oxygen (O2) is the nucleophilic center in methanol and employs one of its lone pairs of electrons to make a new bond to the electrophilic carbon of the acid chloride shown in figure. Since this new bond forms, the carbonyl π bond breaks and both of the electrons move onto the carbonyl oxygen to provide it a 3rd lone pair of electrons and a negative charge (Step 1).
Note: The methanol oxygen gains a positive charge because it has successfully lost an electron by sharing its lone pair along with carbon in the new bond. A positive charge on oxygen is not extremely stable and thus the second stage in the mechanism is the loss of a proton. Both of the electrons in the O-H bond move onto the oxygen to restore a second lone pair of electrons and so neutralize the charge. .
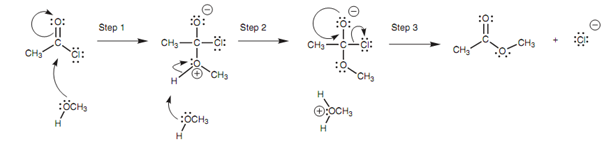
Figure: Mechanism for the reaction of an alcohol with an acid chloride.