SN2 Mechanism (Bimolecular nucleophilic substitution):
The reaction among the methyl iodide and a hydroxide ion is an instance of the SN2 mechanism as shown in figure. The hydroxide ion is a nucleophile and employs one of its lone pair of electrons to make a new bond to the electrophilic carbon of the alkyl halide. At similar time, the C-I bond breaks.
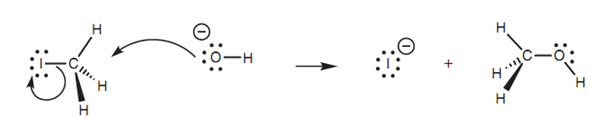
Figure: SN2 Mechanism for nucleophilic substitution.
Both electrons within that bond move onto the iodine to provide it a fourth lone pair of electrons and a negative charge. Because iodine is electronegative, it can stabilize this charge, thus the complete process is favored. In the transition state for this process as shown in diagram, the new bond from the incoming nucleophile is partially created and the C-X bond is broken partially. Itself the reaction center (CH3) is planar. This transition state assists to explain various other features of the SN2 mechanism. Very firstly, both the alkyl halide and the nucleophile are needed to make the transition state which means that the reaction rate is dependent upon both components. Secondly, it can be observed that the hydroxide ion approaches iodomethane from one side whereas the iodide leaves from the opposite side. The iodide ions and hydroxide are negatively charged and will repel each other, thus it makes sense that they are as far apart as possible in the transition state.