Mechanism of acetal formation from a hemiacetal:
Every stage in this mechanism is reversible and thus it is possible to convert the acetal or ketal back to the original carbonyl compound by using water and an aqueous acid like catalyst. Because water is added to the molecule in the reverse mechanism, this is a process termed as hydrolysis.
Acid works as a catalyst both for the creation and the hydrolysis of acetals and ketals, thus how can one synthesize ketals and acetals in good yield? The reply lies in the reaction conditions. While creating acetals or ketals, the reaction is performed in the nonexistence of water by using a small amount of concentrated sulfuric acid or an organic acid like para-toluenesulfonic acid. The yields are further boosted if the water created during the reaction is removed from the reaction mixture. To transform the acetal or ketal back to the original carbonyl compound, an aqueous acid is employed like that there is a large excess of water present and the equilibrium is shifted in the direction of the carbonyl compounds.
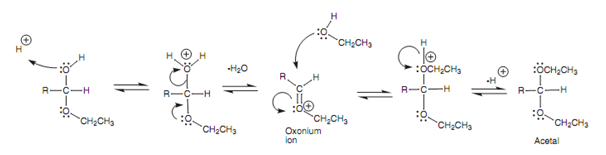
Figure: Mechanism of acetal formation from a hemiacetal.