Hemiacetals and hemiketals:
While aldehydes and ketones are dissolved in alcohol without an acid catalyst being exist, just only the first part of the above mechanism occurs with one alcohol molecule adding to the carbonyl group. Equilibrium is set up among the carbonyl group and the hemiacetal / hemiketal, along with the equilibrium favoring the carbonyl compound as displayed in figure below.
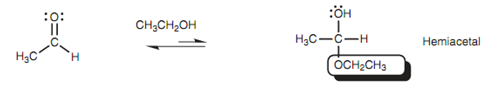
Figure: Hemiacetal formation.
The reaction is not synthetically helpful, because it is not generally possible to isolate the products. If the solvent is eliminated, the equilibrium is driven back to starting materials. Though, cyclic hemiacetals are significant in the chemistry of sugars.