Enamine formation:
The reaction of carbonyl compounds along with secondary amines cannot provide iminessince there is no NH proton to be lost in the last step of the mechanism. Though, there is other way in which the positive charge on the nitrogen may be neutralized. This includes loss of a proton from a neighboring carbon atom as shown in figure. Water works as a base to remove the proton and the electrons that make up the C-H σ bond are employed to create a new π bond to the neighboring carbon. In turn this force the existing π bond among the carbon and nitrogen to break like that both the π electrons end up on the nitrogen atom like a lone pair, thus neutralizing the charge. The final structure is termed as an enamine and can prove helpful in organic synthesis.
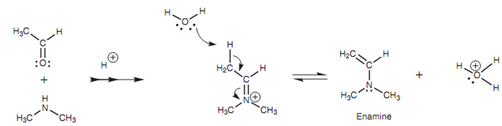
Figure: Mechanism for the formation of an enamine.