Organolithium reagent:
The aldehydes' and ketones' reaction with Grignard reagents is a helpful method of synthesizing primary, secondary, and tertiary alcohols as shown in figure.
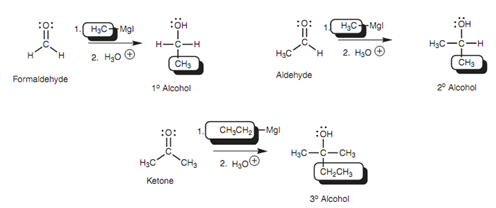
Figure: Synthesis of primary, secondary, and tertiary alcohols by the Grignard reaction.
Primary alcohols can be acquired from formaldehyde, secondary alcohols can be acquired from aldehydes, and tertiary alcohols can be acquired from ketones. The reaction includes the formation of a carbon-carbon bond and thus this is a significant way of building up complex organic structures from simple starting materials.
Grignard reagent itself is synthesized from an alkyl halide and a large range of reagents are probable.
Organolithium reagents like CH3Li can as well be employed to give the nucleophilic carbanion and the reaction mechanism is precisely the same as that explained for the Grignard reaction as shown in figure.
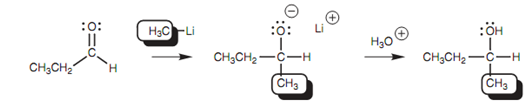
Figure: Nucleophilic addition with an organolithium reagent.