Mechanism for the nucleophilic addition:
The carbanion employs its lone pair of electrons to create a bond to the electrophilic carbonyl carbon. At similar time, the comparatively weak π bond of the carbonyl group breaks and both electrons move to the oxygen to provide it a third lone pair of electrons and a negative charge (Step 1). The reaction ends at this stage, because the negatively charged oxygen is complexed with magnesium that works as a counterion. Aqueous acid is now added to give an electrophile in the shape of a proton. The intermediate is negatively charged and can work like a nucleophile or base. A lone pair of electrons on the negatively charged oxygen is employed to create a bond to the proton and the last product is obtained (Step 2).
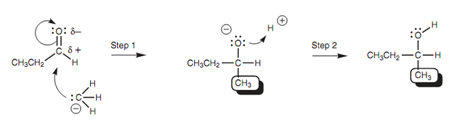
Figure: Mechanism for the nucleophilic addition of a Grignard reagent.