Neutral Inorganic Species:
Polar bonds
If two atoms of relatively different electronegativities are associated together, then the bond connecting them will be polar covalent like that the bonding electrons are biased in the direction of the more electronegative atom. This will provide the latter a little negative charge and build it a nucleophilic center. Alternatively, the less electronegative atom will gain a slightly positive charge and be an electrophilic center. The more to the right an element is in the periodic table, the more electronegative it is. So, ?uorine is more electronegative than oxygen that in turn is more electronegative as compared to the nitrogen.
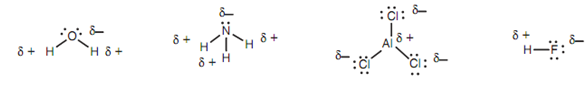
Figure: Nucleophilic (δ -) and electrophilic (δ+) centers in neutral inorganic molecules.
Note: also that all the nucleophilic atoms recognized above have lone pairs of electrons. This is other way of identifying nucleophilic atoms.