Charged Species:
Anions:
A negatively charged molecule like the hydroxide ion that shown in the below diagarm is electron rich and works as a nucleophile. The atom that bears the negative charge and a lone pair of electrons is the nucleophilic center that in the case of the hydroxide ion is the oxygen atom. A number of ions (for example the carboxylate ion) are capable to share the negative charge among the two or more atoms by a process termed as delocalization. In this case, the negative charge is shared among the both oxygen atoms and thus both of these atoms are nucleophilic centers.
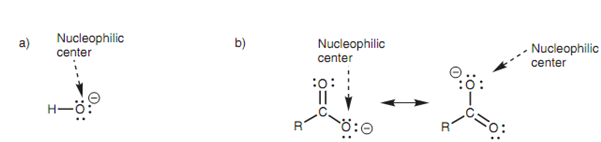
Figure: Examples of nucleophiles; (a) hydroxide ion; (b) carboxylate ion.