Radioactivity
Radioactive decay is a process in which by unstable nuclei change into more stable ones by emitting particles of different types. Alpha, beta and gamma (α, β and γ) radiation was originally categorized according to its distinct penetrating power. The processes involved are demonstrated in the below disgram.
- An α particle is a 4He nucleus and is emitted through some heavy nuclei, giving a nucleus with the Z two units less and mass number four units less. For instance, 238U (Z=92) goes through a decay to give (radioactive) 234Th (Z=90).
- A β particle is an electron. By a nucleus, its emission increases Z by one unit, but does not alter the mass number. So 14C (Z=6) decays to (stable) 14N (Z=7).
- γ radiation contains high-energy electromagnetic radiation. It frequently accompanies α and β decay.
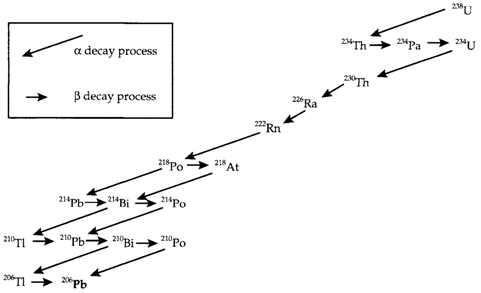
Fig. 1. The 238U decay series showing the succession of α and β decay processes that give rise to many other radioactive isotopes and end with stable 206Pb.
Some other decay processes are known. Extremely heavy elements can decay by spontaneous fission, when the nucleus divides into two fragments of similar mass. A transformation reverse to that in normal β decay occurs either by electron capture by the nucleus or by emission of a positron (β+) the positively charged antiparticle of an electron. So the natural radioactive isotope 40K (Z=19) can go through normal β decay to 40Ca (Z=20), or electron capture to give 40Ar (Z=18).
Radioactive decay is a statistical process, there being nothing in any nucleus which permits us to predict when it will decay. The possibility of decay in a given time interval is the only thing that can be determined, and this appears to be totally constant in time and (apart from in the case of electron capture) not influenced by temperature, the pressure or chemical state of an atom. The probability is generally expressed as a half-life, the time taken for half of the sample to decay. Half-lives can change from a fraction of a second to billions of years. Some naturally taking place radioactive elements on Earth have very long half-lives and are effectively left over from the synthesis of the elements before the formation of the Earth. The most significant of these, with their half-lives in years, are 40K (1.3×109), 238U (4.5×109) and 232Th (1.4×1010).
The occurrence of these long-lived radioactive elements has significant consequences. Radioactive decay gives a heat source inside the Earth, which ultimately fuels several geological processes that includes volcanic activity and long-term movement and generation of the crust. Another elements result from radioactive decay, including argon and helium and various short-lived radioactive elements coming from the decay of uranium and thorium. The Diagram depicts how 238U decays by a succession of radioactive α and β processes, generating other Elements' shorter-lived radioactive isotopes and ending as a stable isotope 206Pb of lead. Likewise decay series starting with 232Th and 235U also produce short-lived radioactive elements and end with the lead isotopes 208Pb and 207Pb, correspondingly.
All elements beyond bismuth (Z=83) are radioactive and none beyond uranium (Z=92) take place naturally on Earth. With increasing numbers of the protons heavier elements have progressively less stable nuclei with shorter half-lives. Elements with Z up to 110 have made up not naturally but the half-lives beyond Lr (Z=103) are too short for chemical investigations to be feasible. Two lighter elements, promethium (Pm, Z=61) and technetium (Tc, Z=43), also have no stable isotopes.
Radioactive elements are made up artificially by bombarding other nuclei, either in the particle accelerators or with neutrons in nuclear reactors. A number of short-lived radioactive isotopes (for example 14C) are produced naturally in small amounts on Earth by cosmic-ray bombardment in the upper atmosphere.