Age-hardening of Aluminium Alloys
In certain alloys precipitation from a single phase may occur. The precipitate phase may be in form of find sub-microscopic particles distributed both around the grain boundaries and throughout the grains. In certain alloys of Al-Cu, Mg-Si and Be-Cu such phases precipitate after suitable heat treatment. These precipitated phases have strengthening effects of the alloys. This hardening of alloys is termed age hardening or precipitation hardening.
Here the process of age-hardening will be described with particular reference to aluminium alloys containing 4% Cu. Figure depicts the equilibrium diagram of Al-Cu system. It is seen that the solubility of Cu in α-phase solid solution decreases steadily and quite considerably with decrease in temperature. At temperature corresponding to point 3, copper forms copper aluminide (CuAl2) which is deposited as coarse particles in and around the grains of α-solid solution. CuAl2 is extremely hard and brittle. If the alloy is now reheated to about 550oC, between the points 2 and 3, CuAl2 is reabsorbed in α-solid solution resulting into single-phase alloy. If alloy from this state is quenched to room temperature, there is insufficient time for CuAl2 to form and Cu atoms are now held in a super-saturated solid solution within the aluminium.
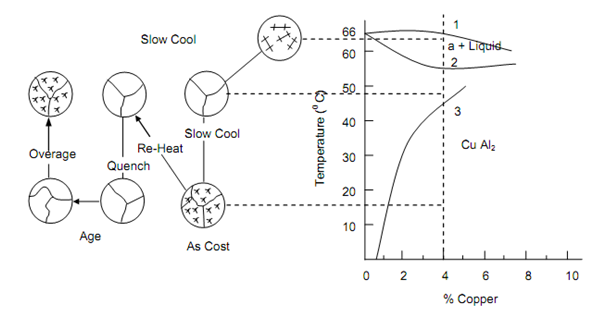
Figure: The Aluminium-rich Portion of the Copper Aluminium Equilibrium Diagram Showing the Mechanism of Precipitation Hardening for a 4% Copper Alloy, Over Aging Causes a Coalescence of the CuAl2 Particles and Consequent Loss of Strength in the Alloy
When this alloy is allowed to stay at room temperature for five to seven days, the strength improves significantly because of slow precipitation of find submicroscopic particles. These particles are almost uniformly distributed around the grains. The time of this precipitation may be reduced to a few hours by heating the quenched alloy to 120oC. This is known as artificial age-hardening. Closed control of both time and temperature is essential in precipitation hardening for this purpose. Salt baths at constant temperatures are used 4% Cu aluminium alloy is most suitable for this type of treatment. However, this alloy loses its corrosion resistance in hardened state and must be protected by cladding.
Age-hardening alloys containing Si and Mg behave in a similar manner. However, the submicroscopic particles that provide strengthening are made of magnesium silicide (Mg2Si). Thus the age-hardening effect of CuAl2 is reinforced by Mg2Si.