Esters:
To name an ester, the following procedure is carried out:
(i) Recognize the carboxylic acid (alkanoic acid) by which it was derived;
(ii) Modify the name to an alkanoate rather than an alkanoic acid;
(iii) Make out the alcohol from which the ester was derived and consider this like an alkyl substituent;
(iv) Name becomes an alkyl alkanoate.
For instance, the ester is derived from methanol and ethanoic acid. The ester would be an alkyl ethanoate because it is derived from ethanoic acid. The alkyl group comes from methanol and is a methyl group. Hence, the full name is methyl ethanoate. (Note: there is a space among the both parts of the name.)
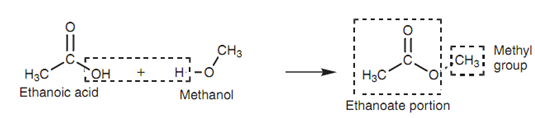
Figure: Ester formation.