Aromatics:
The very well known aromatic structure is benzene. If an alkane chain is related to a benzene molecule, after that the alkane chain is generally considered to be an alkyl substituent of the benzene ring. Though, if the alkane chain consists of more than six carbons, then the benzene molecule is referred to be a phenyl substituent of the alkane chain.
Note: A benzyl group contains an aromatic ring and a methylene group.
Benzene is not the just only parent name that can be employed for aromatic compounds.
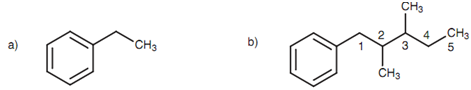
Figure: (a) Ethylbenzene; (b) 1-phenyl-2,3-dimethylpentane
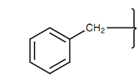
Figure: Benzyl group.
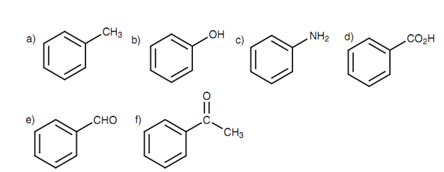
Figure: (a) Toluene; (b) phenol; (c) aniline; (d) benzoic acid; (e) benzaldehyde; (f) acetophenone.