Amines:
The nomenclature for amines is identical to alkyl halides and ethers within that the main part (or root) of the name is an alkane and the amino group is referred to be a substituent. Simple amines are occasionally named via placing the suffix -ylamine later than the main part of the name.
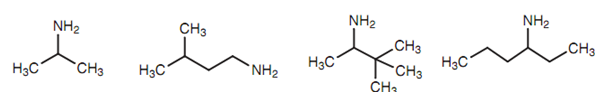
Figure:(a) 2-Aminopropane; (b) 1-amino-3-methylbutane; (c) 2-amino-3,3-dimethylbutane; (d) 3-aminohexane.
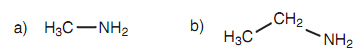
Figure: (a) Methylamine; (b) ethylamine.
Amines comprising more than one alkyl group linked are named by recognizing the longest carbon chain related to the nitrogen. In the instance (as shown in the below diagram), that is an ethane chain and thus this molecule is an aminoethane (N, N-dimethylaminoethane).
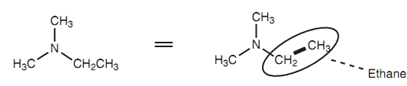
Figure: N, N-Dimethylaminoethane.
A number of simple secondary and tertiary amines have common names.
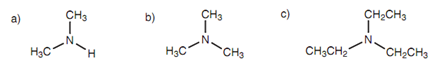
Figure: (a) Dimethylamine; (b) trimethylamine; (c) triethylamine.