Aldehydes and ketones:
For an aldehyde (or alkanal) the suffix is -anal, whereas the suf?x for a ketone (or alkanone) is -anone. The main chain has to involve the functional group and the numbering is like that the functional group is at the lowest number that is possible. The numbering is selected to make sure that other substituents comprises the lowest numbers possible (for example 2, 2- dimethyl-3-pentanone and not 4, 4-dimethyl-3-pentanone), if the functional group is in the center of the main chain. In fact, 3-Methyl-2-butanone can be simplified to 3-methylbutanone. There is just only one possible position for the ketone functional group in this molecule. It would be an aldehyde and not a ketone, if the carbonyl C = O group was at the end of the chain. Numbering is also not essential in locating an aldehyde group because it can just only be at the end of a chain.

Figure: (a) 3-Methyl-2-butanone; (b) 2,2-dimethyl-3-pentanone; (c) 4-ethyl-3-methyl-2- hexanone; (d) 3-methylcyclohexanone.
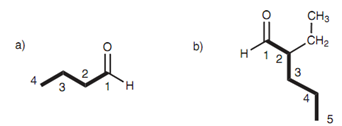
Figure: (a) Butanal; (b) 2-ethylpentanal.