Xenon compounds
The binary fluorides XeF2, XeF4 and XeF6 are thermodynamically stable and can be prepared through direct reaction under suitable conditions. They are very reactive fluorinating agents. The bonding can be explained through three-center molecular orbital pictures or through resonance structures (example 2; see Topic C6) where no valence-shell expansion is needed. The structures of XeF4 (square-planar D4h) and XeF2 (linear) are those supposed in the VSEPR model but that of gas-phase XeF6 has proved elusive. It is supposed that (like predicted for a molecule with a lone-pair) the shape is not a general octahedron, but that fluxional processes lead to a fast interchange among dissimilar distorted configurations. In the solid structure, a few associations among molecules take place and the geometry around Xe is distorted, as supposed in the VSEPR theory.

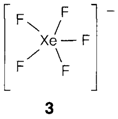
Compounds that come out to consist the [XeF]+ (1) and bent [Xe2F3]+ ions are known even though the former is all the time strongly coordinated to a counterion like BbF6-. Complex anions comprise XeF-5, XeF-7and XeF-8 the first of that has a unique pentagonal planar structure with D5h symmetry (3), as supposed from VSEPR.
Oxohalides like XeOF4 are known. Hydrolysis of XeF6 provides XeO3, that is proportionates in alkaline solution:
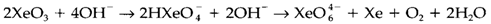
Salts consisting of the octahedral XeVIII perxenate ion XeO4-6are known, and through the action of acid the tetrahedral xenon tetroxide XeO4 is created.
All xenon-oxygen compounds are extremely strong oxidizing and thermodynamically unstable; some like XeO3 are dangerously explosive.
In xenon chemistry currently there has been a renewal of interest, with the preparation of several novel compounds along with Xe-O, Xe-N and Xe-C bonds. Strongly electron withdrawing groups are needed on N and C, an instance being the compound (C6F5)2Xe which such as XeF2 has linear coordination regarding Xe and is made as follows:
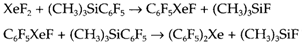
More noticeably, it has been take place that xenon can act like a ligand, and a gold complex consisting of the square planar ion [AuXe4]2+ ion has been prepared.