Oxygen compounds
The most usual encountered oxides, oxoanions and oxocations, are displayed in Fig. 2. All these species contain some multiple bonding, the single N-N and N-O bonds being relatively weak. Nitrous oxide N2O can be created by heating ammonium nitrate. It is isoelectronic with CO2 and rather unreactive, and is employed as an anaesthetic ('laughing gas') and like a propellant for aerosols. Nitrogen dioxide NO2 and Nitric oxide NO are the normal results of reaction of nitrogen and oxygen at high temperatures, or of the oxidation of ammonia. They are both oddelectron molecules. NO2 dimerizes reversibly at low temperatures to create N2O4, but NO has extremely little tendency to dimerize in the gas phase, possibly since the odd electron is delocalized in a π antibonding orbital. NO reacts with oxygen to provide NO2. It can act like a ligand in transition metal complexes. Another oxide of nitrogen is less stable: N2O3 is displayed in Fig. 2. N2O5 is generally found as [NO2]+[NO3]-; and NO3 is an unstable radical that (such as NO and NO2) plays a role within atmospheric chemistry.
NO and (isoelectronic with CO and CO2, correspondingly) can be formed through the action of strong oxidizing agents on NO or NO2 in acid solvents like H2SO4, and are termed as solid salts (example NO+[AsF6]-). The nitrite and nitrate ions NO2- and NO3- are created correspondingly from nitrous acid HNO2 and nitric acid HNO3. As supposed from Pauling's rules, HNO2 is a weak acid in water and HNO3 a strong acid. Metal nitrates and nitrites are strong oxidizing agents, usually extremely soluble in water. Another less stable oxoacids are known, mainly containing N -N bonds. Even though the free acid subsequent to phosphoric acid H3PO4 is unknown, it is feasible to make orthonitrates consisting of the tetrahedral NH3-4 ion. Nitric acid is a main industrial chemical created from ammonia by catalytic oxidation to NO2, followed through reaction with water and more oxygen:
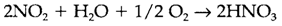
It is employed to make NH4NO3 fertilizer, and in several industrial processes.
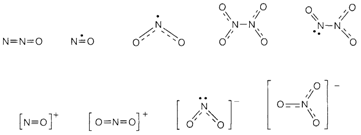
Fig. 2. Structures of some oxides, oxocations and oxoanions of nitrogen.
The nitrogen compounds' redox chemistry in aqueous solution is demonstrated in the Frost picture in Fig. 1. All oxoacids and oxides are strong oxidizing agents and all oxidation states apart from -3, 0 and +5 are susceptible to disproportionation. The comprehensive reactions are, though, mostly controlled by kinetic before thermodynamic considerations. In conjunction with oxidizable groups, like in ammonium nitrate NH4NO3 or in organic nitro compounds, N-O compounds might be powerful explosives.