Other compounds
Compounds with sulfur are explained in Topic F8. Except its fluorides, nitrogen halides are thermodynamically unstable and extremely explosive. The trifluoride NF3 can be prepared through direct reaction of NH3 and F2. It is kinetically nontoxic and inert. Additional fluorination provides the NV species NF4+
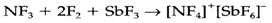
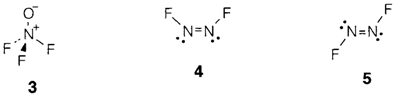
The oxofluoride ONF3 is also well-known. Such as NF4+ it is isoelectronic with NO43-and must be explained by a similar valence structure (3). N2F4 is interesting in that such as N2O4 it eagerly dissociates into NF2 radicals. Double-bonded N2F2 presents in cis (4) and trans (5) forms, the previous being thermodynamically more stable. The point groups are C2v (4) and C2h (5).
Nitrogen reacts straight with some electropositive metals to create nitrides like Li3N and Ca3N2. Even though these can be prepared with nitride ion N3- the bonding might be partially covalent. Other compounds with metals are imides and amides (consisting of NH2-and NH2-, correspondingly) and azides consisting of N3- . Metal azides are thermodynamically unstable and frequently explosive.