Ammonia and derivatives
Ammonia NH3 is build industrially in larger molar quantities than another substance. The Haber process includes direct synthesis from the elements at almost 600 K at high pressure and in the existence of a potassiumpromoted iron catalyst. Ammonia is employed to make nitric acid and other chemicals that are including several plastics and pharmaceuticals.
Ammonia has a C3v pyramidal structure. It is a good Lewis base and a significant ligand within transition metal complexes. In water it acts like a Brønsted base by the equilibrium
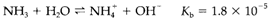
The ammonium ion creates salts and has a identical radius to K+, even though the structures are sometimes dissimilar because NH4+can go through hydrogen bonding. For instance, NH4F has the tetrahedral wurtzite structure than the rocksalt structure of KF; the tetrahedral coordination is perfect for formation of hydrogen bonds among NH4+ and F- ions. Ammonium salts frequently dissociate reversibly on heating:
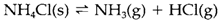
Ammonia has a usual boiling point of -33°C. As with water, this value is very much higher than supposed from the normal group trend, a demonstration of strong hydrogen bonding. Liquid ammonia also goes through autoprotolysis even though to a lesser extent than water. It is a good solvent for several ionic substances, and is much more essential than water. Ammonium salts work as acids and amides as bases. Ammonia is kinetically inert in strongly reducing circumstances, and will dissolve alkali metals to provide solutions with free solvated electrons exist. Hydrazine N2H4 (1) can be created through the Rauschig synthesis:
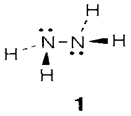
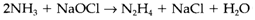
Its combustion to provide N2 and H2O is very exothermic (ΔH=-620 kJ mol-1) and it has been employed as a rocket fuel. The explosive hydrogen azide HN3 is the conjugate acid of the azide ion (2). Other hydrogen compound is hydroxylamine NH2OH.
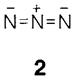
Nitrogen creates an huge variety of organic compounds. Amines like methylamine CH3NH2 and trimethylamine (CH3)3N can be considered as derived from ammonia by replacing one or more H atoms with aryl and alkyl groups. Such as ammonia, amines are essential and form complexes with transition metals. Tetraalkyl ammonium ions like [(C4H9)4N]+ are helpful when large anions are needed in inorganic synthesis. Nitrogen also creates heterocyclic compounds like pyridine C5H5N.