Nitrogen fixation:
The procedure of converting atmospheric N2 gas into ammonia (nitrogen fixation) is carried out through only few microorganisms that termed diazatrophs.
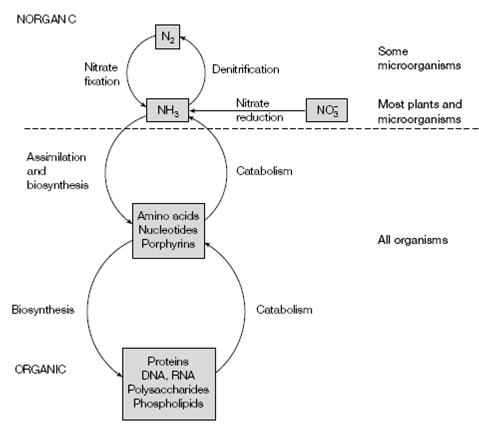
Figure: The interrelationships between inorganic and organic nitrogen metabolism.
These are some free-living soil bacteria like as Azotobacter and Klebsiella, cyanobacteria (blue-green algae) and the symbiotic bacteria (majorly Rhizobium). The symbiotic Rhizobium bacteria invade the roots of leguminous green plants (plants belonging to the pea family, example for beans, clover, alfalfa and form root nodules where nitrogen fixation takes place. The amount of N2 fixed via these diazatrophic microorganisms has been estimated to be in the order of 1011 kg per year, about 60 percent of the earth's newly fixed nitrogen. The Ultraviolet and Lightning radiation fix another 15 percent with the remainder coming from industrial procedures.
The chemical non reactivity of the N≡N bond is clearly seen when one considers the industrial procedure of nitrogen fixation. This process devised through Fritz Haber in the year of 1910 and since used today in fertilizer factories, includes the reduction of N2 in the presence of H2 gas over an iron catalyst at a temperature of 500°C and a pressure of 300 atmospheres.
N2 + 3H2 ↔ 2NH3