Properties:
It is probable to suppose whether molecules are more likely to react like nucleophiles or electrophiles depending upon the strength of the nucleophilic and electrophilic centers present. For instance, ammonia has both electrophilic and nucleophilic centers. Though, it generally reacts like a nucleophile because the nitrogen atom is a strong nucleophilic center and the hydrogen atom is a weak electrophilic center. In difference, molecules like hydrogen ?uoride or aluminum chloride prefer to react like electrophiles. This is since the nucleophilic centers in both of these molecules (halogen atoms) are weak, while the electrophilic centers (H or Al) are strong. Water is a molecule that can react evenly well like a nucleophile or as an electrophile.
For instance, water reacts like a nucleophile along with a proton and like an electrophile with an anion as shown in the below diagram.
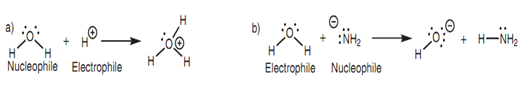
Figure: Water acting as (a) a nucleophile and (b) an electrophile.