Electrophilic strength:
The same argument can be employed in reverse while looking at the relative electrophilic strengths of atoms in dissimilar molecules. Let's compare the electrophilic strengths of the hydrogens in HF, H2O, and NH3. Within this case, reaction with a strong nucleophile or base would produce anions. Fluorine is the most electronegative atom is best capable to stabilize a negative charge and thus the ?uoride ion is the most stable ion of the three. Oxygen is as well able to stabilize a negative charge, though not as well as ?uorine.
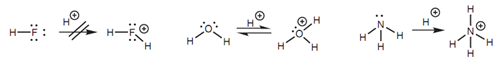
Figure: Cations formed if HF, H2O, and NH3 act as nucleophiles.
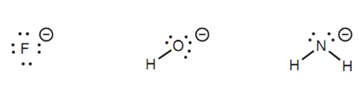
Figure: Anions generated when HF, H2O, and NH3 act as electrophiles.
Nitrogen is the minimum electronegative of the 3 atoms and has the least stabilizing affect on a negative charge and thus the NH2- ion is not stable. The more stable the anion, the more simply it is made and therefore the hydrogen that is lost will be strongly electrophilic. This is the case for HF. In difference, the hydrogen in ammonia is an extremely weak electrophilic center because the anion formed is unstable. The result of it is nitrogen anions are only created with very strong bases.