Decay Series:
Three naturally occurring radioactive decay series/chains corresponding to two isotopes of uranium (235U and 238U) and an isotope of thorium (232Th) were identified immediately after the discovery of radioactivity. These successively decay to give different isotopes of lead by emitting different numbers of α and β particles. 235U (t1/2 = 7.13 × 108 y) decays to 207Pb by emitting 7 α and 4 β particles, 238U (t1/2 = 4.5 × 109 y) decays to 206Pb by emitting 8 α and 6 β particles and 232Th (t1/2 = 1.4 × 1010 y) decays to 208Pb by emitting 6 α and 4 β particles. During successive decay of each of these, different isotopes of elements with Z > 82 are obtained as intermediates. The three series are also called 4n (232Th), 4n+2 (238U) and 4n+3 (235U) as all their isotopes have mass numbers divisible by 4 with remainders as 0, 2 and 3 respectively. Change in mass number (A) in a natural decay chain is entirely due to α-emissions whereas β emission is accompanied through increase in atomic number (Z) but no change in mass number.
Another artificial decay chain called neptunium (237Np, t1.2 = 2.14× 106 y) decay series corresponding to 4n+1 may have existed in the early history of the solar system. Final product of this extinct natural decay chain is 209Bi by emitting 7 α and 4 β particle. Incidentally, end product of all the tree naturally occurring radioactive decay series are lead isotopes ( 206Pb, 207Pb and 208Pb) have Z = 82 which is a magic number. Similarly the end product of artificial readioactive decay series is 209Bi having N = 126 which is again a magic number.
Besides, two weak β activities due to 40K (t1.2 = 1.27× 109 y) and 87Rb (t1.2 = 5.7× 1010 y) were discovered by N.R. Campbell and A. Wood (1906). Later 147Sm (t1.2 = 1.1× 1011 y) was found to emit α - particles. In recent years, several naturally occurring radioactivities with long half lives have been found.
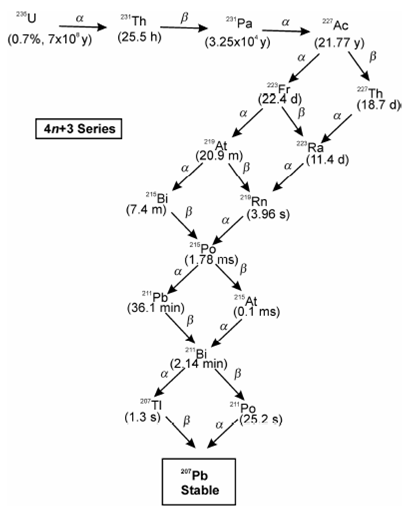
Figure: Natural radioactive decay series of 235U also called 4n+3