The Bohr effect
The binding of O2 to hemoglobin is usually affected by the concentration of H+ ions and CO2 in surrounding tissue; the Bohr effect. In the actively metabolizing tissue, like muscle, the concentrations of the two substances are high relatively.This causes a shift of the O2 dissociation curve for the hemoglobin to the right, promoting the release of O2. This comes about as there are H+ binding sites, primarily His146 in the β-chain, which has higher affinity for binding H+ in deoxyhemoglobin than in the oxyhemoglobin. An increase in CO2 causes an increase in H+ because of the action of the enzyme carbonic anhydrase that catalyzes the reaction:
CO2 + H2O <-------> HCO- + H+
Additionally, CO2 can react with the primary amino groups in polypeptide chain to form the negatively charged carbamate. Again, this change from positive to negative charge favors the conformation of deoxyhemoglobin. On returning in blood to the lungs, concentrations of H+ and CO2 are lower relatively and that of O2 higher, so that the process is made reverse and O2 binds to the hemoglobin. Therefore, it can be seen that not only hemoglobin carry O2 but it carries CO2 back to the lungs where it is expelled.2,3-Bisphosphoglycerate is a highly anionic organic phosphate molecule.
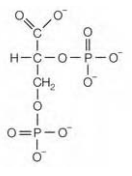
Figure- 2,3-Bisphosphoglycerate
that is it is present in erythrocytes along with the hemoglobin. This molecule promotes release of O2 from hemoglobin by reducing the affinity of the protein for O2. 2,3-Bisphosphoglycerate binds in small cavity in center of four subunits. In oxyhemoglobin this cavity is very small for it, while in deoyhemoglobin it is sufficiently large to accommodate a single molecule of 2,3- bisphosphoglycerate. On binding the central cavity of deoxyhemoglobin it develops ionic bonds with the positively charged amino acid side-chains in β- subunits, stabilizing quaternary structure. H, CO and 2,3-bisphosphoglycerate are all the allosteric effectors as they favor the conformation of deoxyhemoglobin and thus promote the release of O2. As these three molecules act at the different sites, their effects observed are additive.