Hemoglobin
The 3-dimensional structure of hemoglobin was solved using X-ray crystallography in the year 1959 by Max Perutz. This exposed that hemoglobin is made up of four polypeptide chains, each of which has a very similar three- dimensional structure to the single polypeptide chain in myoglobin despite the fact that their amino acid sequences differ at 83 percent of the residues. This highlights a common theme in protein structure: that the different primary sequences can specify similar 3-dimensional structures. The basic type of hemoglobin which is found in adults (HbA) is made up of two types of polypeptide chains: the α-chain which comprises of 141 amino acid residues, and the β-chain of 146 residues. Every chain, as in myoglobin, comprises of eight α-helices and each contains a heme prosthetic group as shown in figure b. Thus, hemoglobin can bind four molecules of O2. The 4 polypeptide chains, two α and two β, are tightly packed together in a tetrahedral array to form a spherically shaped molecule which is held together by multiple noncovalent interactions.
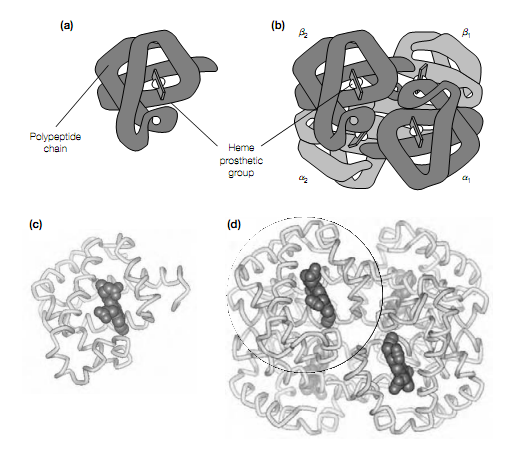
Figure Structure of (a) myoglobin and (b) hemoglobin, showing α and β polypeptide chains. Cα-backbone traces of (c) human myoglobin and (d) human hemoglobin, showing α-helices, heme prosthetic group in space ?lling representation and how monomer of myoglobin maps onto structure of hemoglobin (circle).