Dimethylheptane:
In a number of structures, it is hard to decide which end of the chain to number from. For instance, two different substituents might be located at equal distances from either end of the chain. If that is the matter, the group along with alphabetical priority has to be provided the lowest numbering. For instance, the structure in diagram is 3-ethyl-5-methylheptane and not 5-ethyl-3-methylheptane. Though, there is other rule that might take precedence over the above rule. The structure has ethyl and methyl groups evenly placed from each end of the chain, other than there are two methyl groups to one ethyl group. Numbering should be chosen like that the smallest total is acquired. In this instance, the structure is termed as 5-ethyl-3, 3-dimethylheptane than 3-ethyl- 5,5-dimethylheptane because 5+3+3 = 11 is less than 3+5+5 = 13.
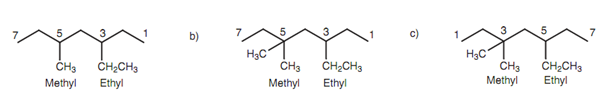
Figure: (a) 3-Ethyl-5-methylheptane; (b) incorrect numbering; (c) 5-ethyl-3,3-dimethylheptane