Ring structures:
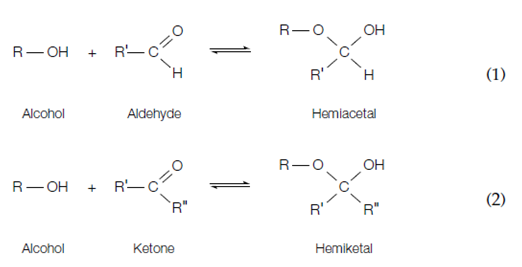
the ketone or aldehyde set can react with a hydroxyl group to form a covalent bond. officially, the reaction between an aldehyde and the hydroxyl group of a sugar an alcohol build a hemiacetal in Equation 1 while a ketone reacts with a hydroxyl group alcohol to form a hemiketal in Equation 2.
For tetroses and larger sugars, the reaction can take place within the similar molecule so which the straight chain form of the sugar cyclizes. For instance, if the C-5 hydroxyl group of glucose reacts with the aldehyde group, a six-membered ring is formed while if the C-4 hydroxyl reacts with the aldehyde group, a five-membered ring is formed. Figure shows the cyclization of D-glucose to form a six-carbon ring. Since of their similarity to the ring compound called pyran, six-membered ring structures are known as pyranoses.
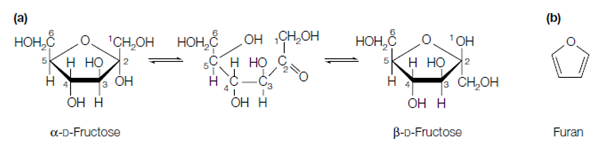
Figure: (a) Cyclization of the open-chain form of D-glucose; (b) the structure of pyran.
The above figure shows the cyclization of the fructose, ketohexose, to form a five membered ring. Because of their connection to the ring compound known as furan, five-membered ring structures are called furanoses. In commonly, ketoses and aldoses with five or more carbons can form either furanose or pyranose rings so which in solution a mixture of these exist. That is the more stable ring form, and therefore predominant, depends on the chemical structure of the monosaccharide, involving the nature of the substituent groups. Commonly aldohexoses like as glucose exist majorly in the pyranose ring form.
The ring structures shown in below figure are known as Haworth projections in that is the plane of the ring can be imagined as around perpendicular to the plane of the paper with the thick lines of the ring in the figure pointing towards the reader. Note that in during cyclization of glucose an aldose, a new
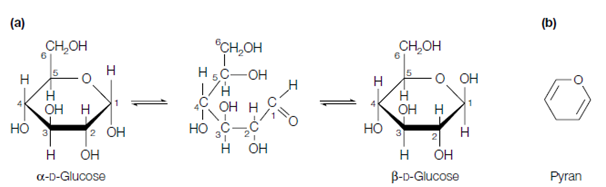
Figure: (a) Cyclization of the open-chain form of D-fructose; (b) the structure of furan
asymmetric center is established, at C-1, the carbon which carried the carbonyl moiety. Therefore two isomers of D-glucose exist, α-D-glucose (in that the OH group at C-1 lies below the plane of the ring) and β-D-glucose (in that the OH group at C-1 lies above the plane of the ring). Pyranose ring structure of β-D-glucose should be written as β-D-glucopyranose. The carbonyl carbon (C-1 in this case) is known as the anomeric carbon atom and so the α and β forms are known as anomers. In aqueous solution the α- and β-forms rapidly interconvert through the open-chain structure, to provide an equilibrium combination. This procedure is known as mutarotation. In the case of the fructose, ketose, the anomeric carbon atom (which carried the carbonyl moiety) is C-2 and thus two isomers (anomers) exist that differ in their configuration about which carbon atom example for. the α/β designation refers to the configuration about C-2 not C-1. Pyranose ring of a six-carbon sugar can exist in either a boat or a chair configuration. The substituents involved to the ring carbons which extend equally to the symmetry axis are said to be axial (a) whilst those which extend outward from this axis are said to be equatorial (e). In the boat form, there is considerable steric hindrance among the several groups attached to the carbon atoms of the ring and thus this form is less favorable energetically. Therefore the chair form predominates, as shown for β-D-glucose in Figure, where all the axial positions are occupied through hydrogen atoms.
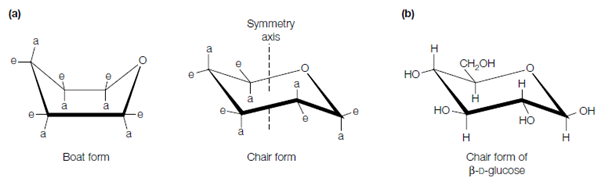
Figure: (a) Chair and boat conformations of pyranose rings; (b) stable chair form of -D-glucose.